
There are two main types of reactions responsible for the browning process:
1. Maillard reaction: a very complex set of reactions starting with an amino acid reacting with a sugar. I will explore this reaction in another post since it is omnipresent in cooking.
2. Pyrolysis: a complex series of reactions starting with the spontaneous breaking down of either carbohydrates or proteins due to high temperatures. Caramelization (oxidation of sugars) is a type of pyrolysis. Like with Maillard reactions, flavour and colour compounds are formed.
While both reactions are involved in browning foods, the Maillard reactions dominate by a large margin. The extent of each reaction depends on temperature, sugar content, protein content, etc.
Chefs and cooking enthusiasts often throw out terms like caramelization and the Maillard reaction, without differentiating the two. There seems to be a great deal of misunderstanding and confusion among online communities. The key point to remember is that both pyrolysis and Maillard reactions are involved, but it is the Maillard reactions that dominate.
I've recently read online posters claiming that Maillard reactions are not involved with the browning of meat due to the lack of sugars. They propose instead that the main player is pyrolysis. I have not been able to find a citation to support this. In fact, I have found experimental evidence of the contrary (see reference 2 at the end of the post). The problem with this claim is that there is an abundance of sugar in meats, as part of molecules found in cells (ATP, nucleic acids). Scientific literature I have read state that ribose, the sugar found in ATP and nucleic acids, is the major sugar participant in the Maillard reaction in the browning of meat. Hence, there's no reason to doubt there is enough sugar in meat for Maillard reactions.
So how does the browning process begin? With sufficient heat! The reactions involved occur most readily, or sometimes exclusively, at very high temperatures.
Heat is not the only requirement though. Food does not brown when it is boiled or steamed, even though there is a heat source. This is due to the presence of free water (ie: not water content in the meat). Non-enzymatic browning requires temperatures in excess of 100°C (boiling point of water). That means when a lot of water is present in liquid form, no browning can occur because water will take up all the heat and keep temperatures from exceeding 100°C. I drew up a little diagram in photoshop, mostly for fun.
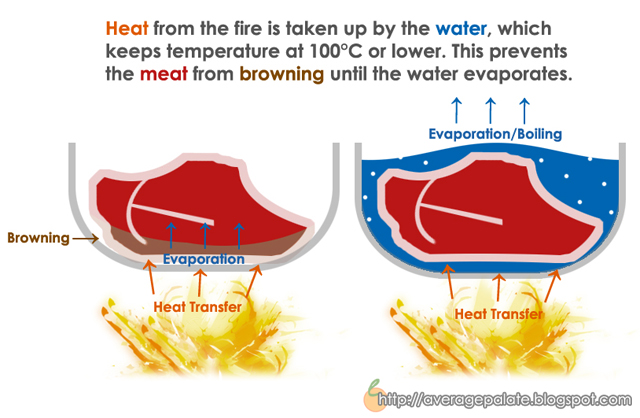
You might be wondering about the inherent water content in food. Since there's always water in food, why does browning occur at all? The answer is simple when we think about how it's only the exterior of what we are cooking that is browned. What happens is that as water evaporates from the food's surface, the temperature can increase increase to sufficient browning levels.
There's another reason why water inhibits the browning process besides temperature. The Maillard reaction produces water. If water is already present in the system (ie: your pot or pan), a reaction that produces water is less likely to occur. (Remember Le Chatelier's principle from high school chemistry?)
For the scientifically inclined:
Mottram DS (1998). Flavour formation in meat and meat products: a review. Food Chemistry 62(4), 415-424.
Pearson AM, Tarladgis BG, Spooner ME & Quinn JR (1966). The Browning Produced on Heating Fresh Pork II. The Nature of the Reaction. Journal of Food Science 31(2), 184-190.








No comments:
Post a Comment